RADIANT (Radiotherapy Assessment During Intervention ANd Treatment) is an observational research study exploring the relationship between genomic variability in individuals and their response to radiation therapy. The primary goal is to confirm the results of an earlier 81-patient study which showed that gene expression analysis of a pre-radiotherapy blood sample can predict therapy response as well as which patients will experience grade 2 or higher toxicity (CTCAE 4.03).
Exploring Radiotherapy Response & Toxicity

About 500 adult cancer patients nationwide

Primary gastrointestinal, genitourinary, or gynecological cancer and planned radiation treatment to pelvic/abdominal area

Self-reported health history and treatment experience via ePRO

Blood samples before, during, and after radiotherapy. All samples will be taken via DxCollect MCD Fingerstick Kit.

With treating oncologist to introduce RADIANT and verify treatment information
How does RADIANT work?
RADIANT uses a direct-to-patient (D2P) clinical study platform to remotely engage patients and physicians in observational research from the comfort and convenience of home through a secure environment. The D2P approach uses a combination of physician referrals and direct digital recruiting to find eligible patients. Study participants provide digital consent for the study along with consent to obtain their medical records and contact their physician. The participants provide self-collected fingerstick blood samples for genomic analysis as well as completing short, weekly electronic Patient Reported Outcome (ePRO) questions.
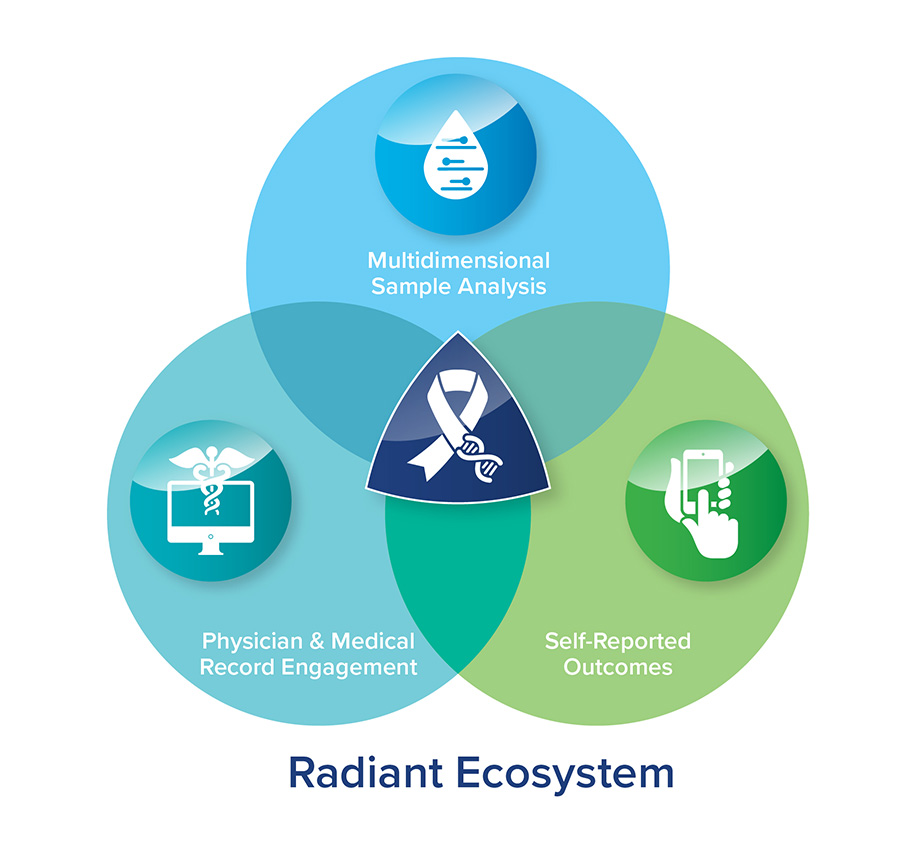
Integrating self-reported data, physician engagement, medical records, and blood samples, to optimize genomic analysis outcomes.
1. Samples and data may be self-collected from participants
2. Treating physicians and medical records are consulted, providing clinical context of self-reported data
3. Samples are sent to DxTerity for gene expression analysis
4. Gene expression data is correlated back to the patient’s response to radiation treatment
D2P expands clinical studies to anyone, anytime, anywhere, potentially lowering the cost while expanding the size of patient cohorts and resulting in a more efficient and cost-effective solution for validating a promising genomic test.
An important and scientifically critical aspect of RADIANT is obtaining physician engagement. Connecting treating physicians to RADIANT helps verify and validate self-reported outcomes and maintain scientific integrity. We invite physicians to participate by answering a few questions about their patient’s health history and treatment course.